Introduction about visual evoked potentials {VEP}
- Iatrogenic injury to visual function is a major complication of brain surgery, while resection of intrinsic brain tumors that are close to either the optic nerves, visual tracts, or occipital cortices can cause deterioration of visual function. Typically these type of complications usually can occur in procedures for tumors of or around optic nerves and also temporal lobectomies either for epilepsy surgery or for temporal neoplasms. Brain surgeries for parietal and occipital lesions associated with the optic radiations or the visual cortex also carry an intrinsic risk of visual deficit .
History.
- In 1973, Wright et al. reported the first case in which flash VEP monitoring was used during surgery under general anesthesia for an intraorbital tumor. The clinical use of flash VEP monitoring under general anesthesia for preservation of visual function was subsequently investigated, but no clear utility was observed.
- Because the potential was obtained under general anesthesia, using the evoked potential measurement equipment available at that time was unstable and showed poor reproducibility that weakened the relationship between changes in the potential and postoperative visual function. Intraoperative flashVEP monitoring eventually stopped being used in clinical settings around 1990 as an intraoperative tool , however, several recent breakthroughs have rekindled its use under general anesthesia.
- The first was the spread of total intravenous anesthesia using propofol, which reduced VEP suppression by anesthetics. This was followed by the development of light-emitting diodes(LEDs) with strong illuminance, photo-stimulation devices using LEDs allowed the retina to be more strongly photo-stimulated.
In current Era.
- In recent years the use of total intravenous anesthesia (TIVA) the incorporation into the hardware of light emitting diode (LED) technology and adjuncts such as electroretinography (ERG) have attempted to overcome. technical setbacks previously encountered in VEP neuromonitoring enhancing the technique’s reproducibility and interpretability, and working to make it more reliable and easier to integrate into the operative workflow.
Indication.
- Use of intraoperative VEP monitoring during trans sphenoidal pituitary surgery despite the close relationship that this surgical approach maintains with the optic apparatus. Intuitively the technique is best indicated for surgeries involving tumors of the anterior skull base that are particularly adherent to the optic chiasm and nerves such as craniopharyngiomas or meningiomas.
Intraoperative Neurophysiological Monitoring.
- If patients undergoing trans sphenoidal surgery for pituitary adenoma or any other type of cases like wherever the risk of visual tract injury, intraoperative visual evoked potential monitoring is a safe, reliable and effective technological adjunct in intraoperatively alerting the surgeon of compromise to the visual pathway and in predicting post-operative visual outcome.
- There is consensus that VEP recordings can only be obtained in patients without severe visual impairments .
- Shall not be able to record or may be able to record delayed latency with low amplitude in case of preoperative Visual Evoked Potentials in patient with moderate to severe preoperative visual impairment.
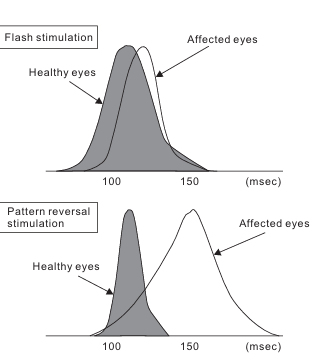
- Comparison of the average latency of major negative vertices of VEP induced by flash stimulation and pattern reversal stimulation showing both delayed time interval usually happens in moderate visual function impairment . incase of severe visual function impairment won’t be able to record good amount of potentials.
VEP stability and reproduciblity are also affected by non patient factors and particularly important techniques to augment this are the use of total intravenous anesthesia {TIVA}, the use of LED goggles or silicone discs for light stimulus delivery, a black shield patch can be placed over the eyes and braided electrode cables, and also can be preferred as a second tool of simultaneous electroretinography (ERG) monitoring .
Usually VEP is divided in two type .
- Neurophysiological Monitoring or test divided in two major parts one subjective and another is objective , according to Visual Evoked Potential pattern-ship VEP is subjective because patients needs to watch the screen while doing this test, And another is objective like flash VEP just only fix the LED goggle over the close eye ,what we do in surgery under General Anesthesia.
- VEP is can be obtained from the visual cortex by applying photo stimulus to the retina, which is exposed to flash stimulation or pattern reversal stimulation The neurons of the visual cortex are highly sensitive to visual stimuli by graphics with contours and contrast.
- Pattern reversal stimulation involving black and white lattices exchanging position at regular intervals was developed using this principle, and is excellent for effectively stimulating neurons of the visual cortex.
- However pattern reversal stimulation cannot be performed under general anesthesia therefore, flash stimulation will performed, whereby a strong light would be delivered to the retina.
Brief about Anatomy and physiology of the optic pathway.
- To understand VEP, knowledge regarding the anatomy and physiology of the optic pathway is required.
- That is, it is important to know which parts of the optic pathway can be damaged by surgical manipulations and what symptoms will develop as a result.
- The optic pathway by which visual information is transmitted travels from the retina to the optic nerve, optic chiasm, optic tract, lateral geniculate body, optic radiation, and visual cortex of the occipital lobe. Visual images (light information) are converted into nerve signals in the retina and are transmitted to the brain via the optic nerve.
Retina.
- Light that enters the eye is sensed by the photoreceptor cells (rods and cones) present in the retina after which it is converted into nerve signals and subjected to complex processing by various nerve cells in the retina until the information is finally transmitted from ganglion cells in the center of the retina to the optic nerve.
- The retina is formed by seven layers from the interior to the exterior the ganglion cell layer, inner plexiform layer, inner nuclear layer, outer plexiform layer, outer nuclear layer, photoreceptor layer, and pigmented epithelial layer.
- The photostimulus that reaches the retinal surface stimulates primary neurons (photoreceptor cells) in the outer nuclear layer and photoreceptor layer, and this stimulation is transmitted to secondary neurons (bipolar and horizontal cells) and tertiary neurons (ganglion and amacrine cells), which transmit the information from the optic nerve to the axons of the ganglion cells and to the central nervous system.
Optic chiasm, lateral geniculate body, and visual cortex.
- Light information input into the nasal retina propagates to the contralateral lateral geniculate body by intersecting at the optic chiasm.
- Meanwhile, light information input to the temporal retina propagates to the ipsilateral lateral geniculate body. The lateral geniculate body contains almost no neurons that receive information from both eyes. The synthesis of information from the left and right eyes first occurs in the cerebral cortex.
- The visual cortex that receives information from the lateral geniculate body corresponds to Brodmann areas 17, 18, and 19 of the occipital lobe.
- A large part of the primary visual cortex of the human brain (Brodmann area 17) is embedded in the medial aspect of the occipital lobe.
- The conduction pathway to Brodmann area 17 reaches this area from the lateral geniculate body via the optic radiation. Information conduction pathways to Brodmann areas 18 and 19 contain input from Brodmann area 17 direct input from the lateral geniculate body bypassing the optic radiation and input from the superior colliculus and pulvinar of the thalamus bypassing the lateral geniculate body In addition, hemispherical coupling occurs between neurons in Brodmann areas 18 and 19 via the corpus callosum.
stimulation technique.
Because patients are unable to cooperate under general anesthesia, flash stimulation-induced VEP is the only method capable of objectively evaluating visual function.
High-intensity LEDs are embedded in the flash stimulation pad, and the small disc shape and silicone properties of the pad make it both flexible and lightweight.
Illuminance can be set up to 20,000 lux, and different light emission times and cycles can be chosen.
The flash stimulation pad can be placed over both eyelids with the eyes closed and fixed with cornea-protecting tape or another fixative to prevent it from dislodging.
Covering the pad with alight-shielding sheet is an effective means of preventing light,
such as surgical lighting, from entering when performing flash stimulation.
Flash stimulation can be performed at a frequency of less than 4 Hz, but is generally performed at around 1 Hz. When the frequency exceeds 5 Hz, the sinusoidal steady-state VEP would be recorded, and when the stimulation frequency if further increase, the amplitude gradually decreases according to signal changes of waveform, (threshold frequency). can be set to throughout the procedure.
The flash stimulation can be preferred to set an intensity that is slightly higher than that at which the VEP amplitude is attenuated by decreasing the stimulation intensity from 20,000 lux to perform maximum stimulation.
Recording.
- Electrode placement corkscrew electrodes to be placed on the patient’s scalp according to the international 10-20 system or other standardized electrode placements for visual evoked potentials. The active electrode is typically placed over the occipital region (Oz), with reference and ground electrodes placed at appropriate locations.
- Grounding and impedance check, Electrode impedance should be checked and maintained at low levels {5kilo ohm’s} to ensure optimal signal quality. Proper grounding of the recording system is essential to minimize noise and interference.

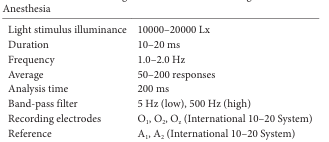
Settings for Flash VEP Monitoring under General Anesthesia.
Intraoperative assessment of VEP.
- Flash stimulation induced VEPs are evaluated by examining the peak-to-peak amplitude between negative wave near 75 milliseconds (N75) and positive wave near 100 milliseconds (P100) When the peak-to-peak distance between N75 and P100 decreases by at least 50% from the baseline amplitude, this is immediately can reported to the surgeon as a significant change in the VEP .
- However as the VEP amplitude if low at least two or more recordings of the same waveform must be confirmed to verify reproducibility .
- The continuous disappearance of VEP waveforms can be interpreted as the onset of severe postoperative visual impairment.
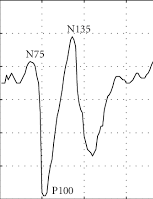
Flash VEP waveform.
Flash VEP is evaluated by examining the peak-to-peak amplitude between negative wave near 75 milliseconds (N75) and positive wave near 100 milliseconds (P100). A significant decrease in flash VEP is defined as a decrease in peak-to-peak distance between N75 and P100 by at least 50% from the baseline amplitude.
Cause that Affect Flash VEP.
Preoperative visual function.
- In patients with severe visual impairment prior to surgery VEP waveforms have low reproducibility and are difficult to record.
Core temperature.
Cause to the effects of hypothermia on VEPs caution is required during general anesthesia when changes in body temperature are prone to occur.
Synaptic transmission is more susceptible to the effects of hypothermia than axial propagation.
A drop in temperature by 1°C reduces in peripheral conduction by 5% and central conduction by 15% will show delayed latency.
partial pressure of carbon dioxide in the blood
- In hypocapnia changes occur in pH, the concentration of ionized calcium and the ion balance of nerve membranes that promote the stimulation of neurons.
Hypoxia and hypotension,
- Compared to the spinal cord and subcortex, the cerebral cortex has a high metabolic rate that gives it a low tolerance to hypoxia .
- Furthermore decreases in the mean arterial blood pressure that go beyond the scope of autoregulation affect the evoked potential because the transport of oxygen to neurons is reduced.
Hemodilution.
- Blood is diluted by maintenance of normovolemia using crystalloid and or colloid replacement for intraoperative hemorrhage. Excessive hemodilution can change VEPs.
Displacement of electrode.
- If the LED flash stimulation electrode is dislodged from its position due to turning of the skin flap the illuminance that reaches the retina becomes insufficient and flash VEP monitoring becomes difficult.
Anesthesia agents which affect neural activity and sensory processing can influence the amplitude, latency and overall characteristics of VEP waveforms.
Inhaled Anesthetics Isoflurane, Sevoflurane ,Desflurane ,N2O .
- These agents exert their effects primarily by enhancing inhibitory neurotransmission and suppressing excitatory neurotransmission in the central nervous system.
- Inhaled anesthetics can decrease the amplitude of VEP responses by attenuating neuronal excitability and reducing synaptic transmission in the visual cortex.
- They may also prolong VEP latency due to delayed neural conduction along the visual pathway.
Intravenous Anesthetic Propofol, Thiopental etc.
- Intravenous anesthetics act on various neurotransmitter systems, including gamma-aminobutyric acid (GABA) receptors, to produce sedative and anesthetic effects.
- These agents can suppress VEP responses by inhibiting cortical activity and reducing neuronal firing rates in the visual cortex.
- Intravenous anesthetics may lead to a decrease in VEP amplitude and prolongation of VEP latency.
Opioids Fentanyl, Remifentanil ,Morphine.
- Opioids primarily act as agonists at mu-opioid receptors in the central nervous system, resulting in analgesia and sedation.
- Bolus dose of opioids can affect VEP signal by attenuating cortical arousal and modulating pain perception, preferable constant infusion like other TIVA Drugs.
- While opioids may not have a direct impact on VEP waveform characteristics, they can indirectly influence VEP responses by altering overall neural excitability and arousal levels.
Neuromuscular Blocking Agents Rocuronium, Vecuronium ETC.
- Neuromuscular blocking agents typically do not have any effect on VEP waveform parameters because these drugs act on NMJ .
- Also will be useful to avoid any muscle activity.
Local Anesthetics.
- Local anesthetics, such as lidocaine or bupivacaine, are often used for regional anesthesia to provide analgesia and block sensory nerve transmission.
- While local anesthetics primarily target peripheral nerves, systemic absorption and distribution can potentially affect central nervous system function, including visual pathway processing.
- However, the direct impact of local anesthetics on intraoperative VEP monitoring is less pronounced compared to other anesthesia agents.
Below this image trying to show how does affect Anesthesia drugs and how to titrate the drug dose.

How does affect sevoflurane and propofol on flash VEP waveform.
- (A) Sevoflurane markedly suppresses flash VEP waveform by extending VEP latency and reducing VEP amplitude in a concentration dependent manner even at low concentrations.
- (B) Propofol has less suppressive effects on the flash VEP waveform, However even propofol suppresses the flash VEP when administered in high doses.
Conclusion.
Previous it was difficult to record stable flash VEP waveforms under general anesthesia recent developments, including propofol other neutral drugs like Dexmedetomidine, etomidate anesthesia, retinal flash stimulation devices using high intensity LEDs, and a combination with ERG monitoring
to confirm that the flash stimulus has reached the retina, have made it easy to obtain reproducible flash VEP waveforms under general anesthesia.
Relatively major postoperative visual impairment can be detected by intraoperative decreases in the flash VEP amplitude.
{This article will be useful to understand the overall concept the technique and their theory, how to record and interpretate the real time signal while surgeon working on or around the visual pathway.
Related to this article.
https://neurointraoperative.com/wp-admin/post.php?post=1502&action=edit
https://pubmed.ncbi.nlm.nih.gov/29600911
Question.
Why we only prefer Flash Visual Evoked Potentials in intraoperatively?.
How much can affect General Anesthesia Drugs?
Which is suitable Anesthesia Drugs to record Flash VEP modality while procedure being performed?.
How much can affect, Hypoxia and Hypotension or other physiological factors. {signal of potentials}.
What is the morphology of VEP signal?.
